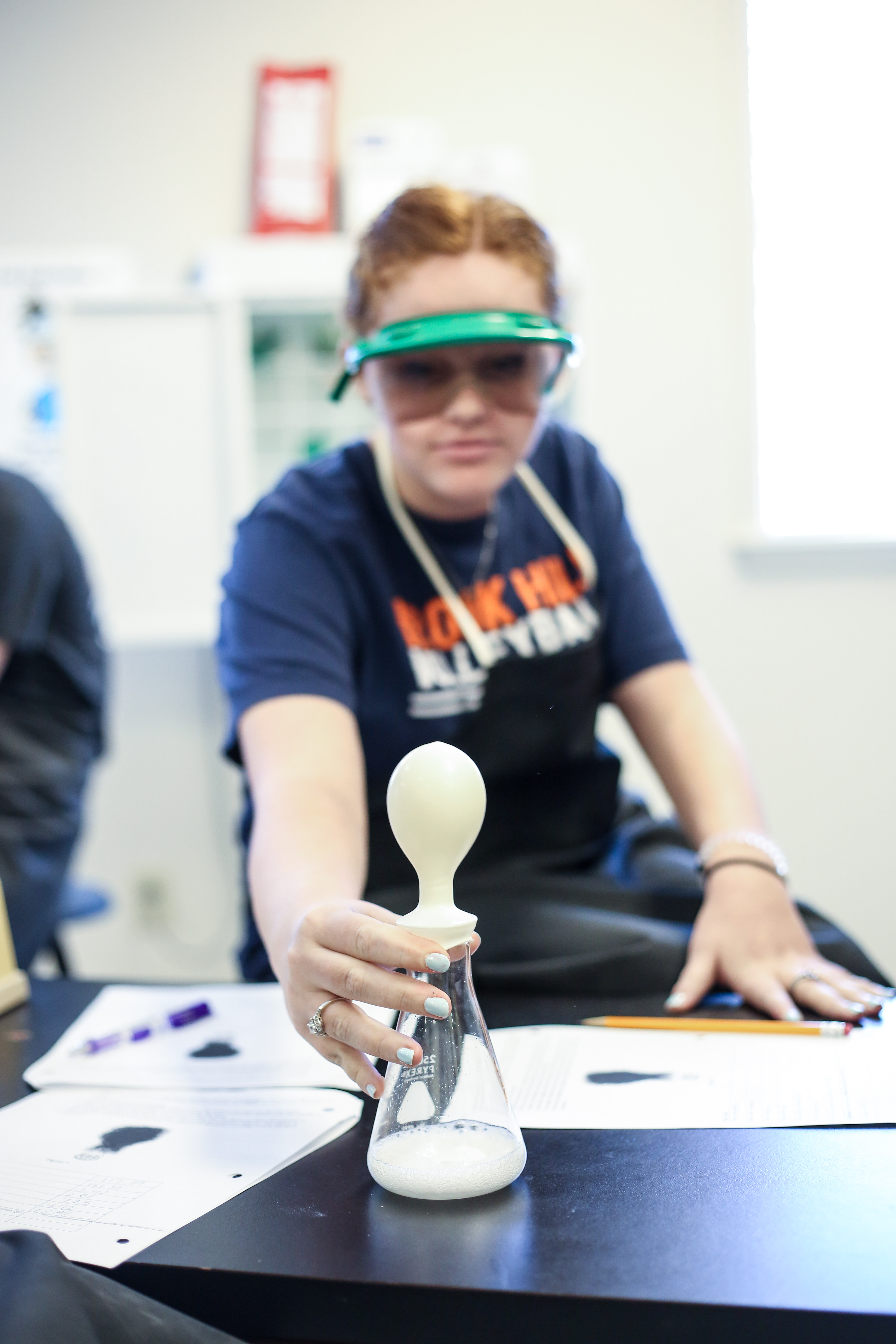
Chemistry Lab: Conservation of Mass
Did you ever do the volcano science fair project? Chemistry students recently did a mild variation of that experiment to study the law of conservation of mass. They did two trials of the baking soda and vinegar concoction, weighing the products and reactants each time. In the first trial, the apparatus was open, allowing the gaseous carbon dioxide product to escape, while the other was closed, collecting the gas so its mass could be determined. What do you think their data showed? I suppose the title of the lab gives it away!